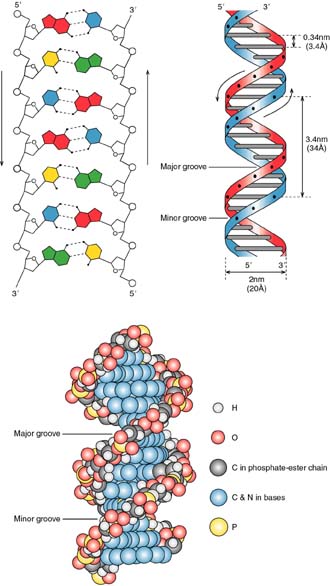
The image of the DNa double helix is practically iconic in our culture. This structure, as you all know, was propsoed by James Watson and Francis Crick in their classic paper in nature (click here for a copy of their original short 1953 paper). The double helix is represented in three ways in Figure 2.14:

There are several key features of this structure:
1. The two strands are held together by two forces:
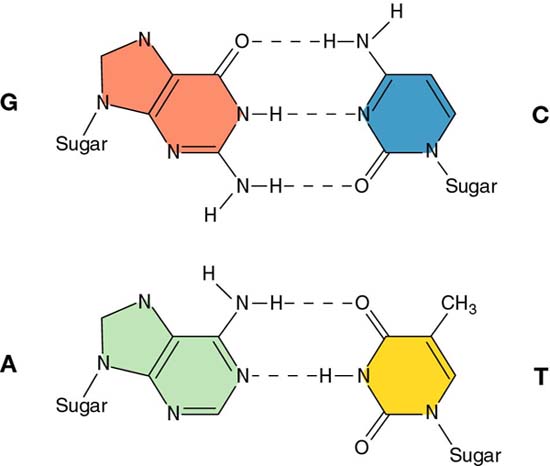
a. the energy of hydrogen bonding between the complementary bases (A=T and G=C)
Figure 2.13
|
An AT Base pair |
GC Base Pair |
 |
 |
b. the hydrophobic effect. While the edges of the base pairs can make hydrogen bond contacts, the planar surfaces are relatively hydrophobic. Therefore, water is less ordered with the bases outside the helix (no base-paired) than when they are inside (base-paired). The hydrophobic effect that produces this arrangement is called base stacking.
2. The two strands are antiparallel.
That is one strand goes in the 3' to 5' direction, while its complementary strand goes in the opposite direction. This is shown in Figure 2.14, above.
3. The helix has three distinct forms:
| A Form | B Form | Z Form | RNA-DNA Hybrid | |
|---|---|---|---|---|
| Direction of helix rotation | right | right | left | right |
| Residues per turn | 11 | 10 | 12 | 11 |
| Rotation per residue | 33 deg. | 36 deg. | –30 deg. | 33 deg. |
| Rise | 0.255 nm | 0.34 nm | 0.37 nm | 0.255 nm |
| Pitch | 2.8 nm | 3.4 nm | 4.5 nm | 2.8 nm |
A- form DNA occurs when the amount of water in the surrounding medium is about 75%. The B- form occurs at much higher moisture content, 92%. Z-DNA, or left-handed DNA occurs in regioins of high GC content. Notice that an RNA/DNA hybrid has the same structural parameters as A form DNA.
Here's a side-by-side comparison of A-form and B-form DNA, using wire-frame models:
4. The surface features of B-form DNA have distinct major and minor grooves.
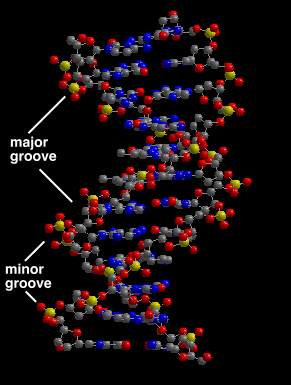
These surface features will become important when we consider how regulatory proteins interact with DNA sequences.