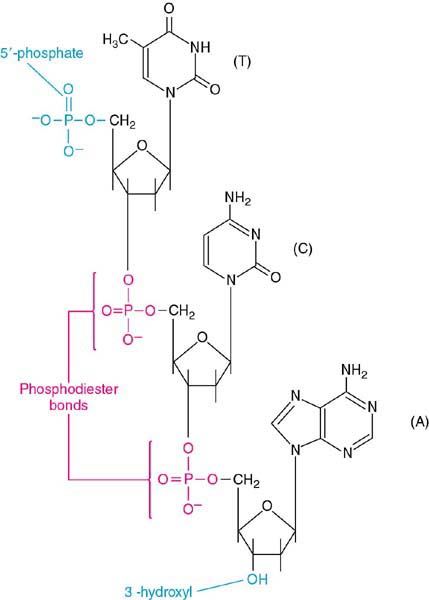
The Phosphodiester Bond
Nucleic acids (RNA and DNA) are formed by the condensation of
nucleotides, catalyzed by polymerases. The bond that is formed is called a
phosphodiester bond. The bond is shown in Figure 2.10, a display of a
trinucleotide DNA fragment.
|
Notice that the bonds in this case are formed between
the 3' carbon of one sugar and the 5' carbon of the next sugar. If we
follow the phosphodiester backbone of this trinucleotide from top to
bottom, we see that there is a direction. That is, we begin at the
phosphate on the 5' carbon and go 5' - 3' along the backbone. An
understanding of this direction is critical to your success in molecular
biology. Make sure that the chemistry of these structures is clear to you
and that this directionality is apparent. |
|
Rather than draw out all of these structures, we use a couple of
shorthand notations to designate nucleic acid polymers. One form of notation
uses vertical lines to represent the sugars and diagonal lines to represent the
phosphodiester bonds (Figure 2.11)

More commonly, we need only indicate the order of bases and the
direction of the polymer chain. For instance:
5' - AGTCCGATGCAAGCTCG -
3'
Can you write the complementary strand that would make this a double
helix?
Here
is my answer: